Drug discovery
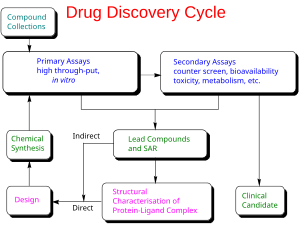
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.
Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy.
Modern drug discovery involves the identification of screening hits, medicinal chemistry and optimization of those hits to increase the affinity, selectivity (to reduce the potential of side effects), efficacy/potency, metabolic stability (to increase the half-life), and oral bioavailability. Once a compound that fulfills all of these requirements has been identified, the process of drug development can continue. If successful, clinical trials are developed.
Modern drug discovery is thus usually a capital-intensive process that involves large investments by pharmaceutical industry corporations as well as national governments (who provide grants and loan guarantees). Despite advances in technology and understanding of biological systems, drug discovery is still a lengthy, "expensive, difficult, and inefficient process" with low rate of new therapeutic discovery. In 2010, the research and development cost of each new molecular entity was about US$1.8 billion. In the 21st century, basic discovery research is funded primarily by governments and by philanthropic organizations, while late-stage development is funded primarily by pharmaceutical companies or venture capitalists. To be allowed to come to market, drugs must undergo several successful phases of clinical trials, and pass through a new drug approval process, called the New Drug Application in the United States.
Discovering drugs that may be a commercial success, or a public health success, involves a complex interaction between investors, industry, academia, patent laws, regulatory exclusivity, marketing and the need to balance secrecy with communication. Meanwhile, for disorders whose rarity means that no large commercial success or public health effect can be expected, the orphan drug funding process ensures that people who experience those disorders can have some hope of pharmacotherapeutic advances.
Comments
Post a Comment